These educational webinars feature a range of topics to help patients and caregivers learn more about their treatment options. If you have questions about ALS, RADICAVA ORS® (edaravone), or RADICAVA® (edaravone) IV, call a JourneyMate Specialist for answers toll-free at 1-855-457-6968.
TO LEARN MORE ABOUT RADICAVA®, SPEAK WITH A JOURNEYMATE RESOURCE SPECIALIST 1-855-457-6968. SEE MORE
TO LEARN MORE ABOUT RADICAVA®, SPEAK WITH A JOURNEYMATE RESOURCE SPECIALIST 1-855-457-6968. SEE MORE
Educational Webinars for ALS Patients
A Journey With RADICAVA ORS® (edaravone)
Watch ALS advocate Jodi O’Donnell Ames interview person with ALS Stacy Lewin, MD, about her journey with ALS and RADICAVA ORS® treatment.
Review helpful information about RADICAVA ORS® and RADICAVA® IV, and watch the How RADICAVA® May Help video.

A Journey With RADICAVA ORS® (edaravone)
Get to Know RADICAVA ORS® (edaravone) for ALS
Hear ALS Caregiver Advocate Jodi O'Donnell-Ames and Senior Marketing Director at MTPA Lia Thornton discuss supporting the ALS community, how RADICAVA ORS® treatment can help, what happens after an ALS diagnosis, and educational resources. They also answer some common questions about RADICAVA ORS®.
Review helpful information about RADICAVA ORS® and RADICAVA® IV, and watch the How RADICAVA® May Help video.

Get to Know RADICAVA ORS® (edaravone) for ALS
Understanding Amyotrophic Lateral Sclerosis (ALS) with Dr Jeffrey Rosenfeld, MD, PhD
Review helpful information about RADICAVA ORS® and RADICAVA® IV, and watch the How RADICAVA® May Help video.

Understanding Amyotrophic Lateral Sclerosis (ALS) with Dr Jeffrey Rosenfeld, MD, PhD
Conversations About RADICAVA ORS®: A Clinical Coordinator's Perspective
ALS Clinic Coordinator Tara Charlton shares her experience working in a clinic, including what you can expect during your first visit and insurance and financial support options that may be available.
Review helpful information about RADICAVA ORS® and RADICAVA® IV, and watch the How RADICAVA® May Help video.
Conversations About RADICAVA ORS®: A Clinical Coordinator's Perspective
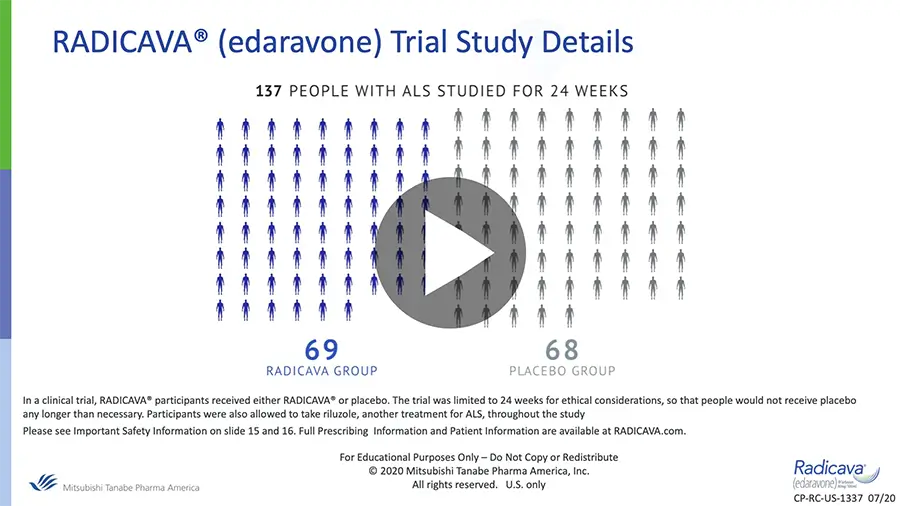
Efficacy and Safety of RADICAVA®
Dr. Joshua Alpers discusses the clinical trial results for RADICAVA®, including Important Safety Information.
Review helpful information about RADICAVA ORS® and RADICAVA® IV, and watch the How RADICAVA® May Help video.

Efficacy and Safety of RADICAVA® Presented by Dr. Joshua Alpers
Introduction to RADICAVA® for Newly Diagnosed Patients
A Senior Medical Director at Mitsubishi Tanabe Pharma America presents information on RADICAVA®, reviewing your options for getting RADICAVA®, and other available resources
Review helpful information about RADICAVA ORS® and RADICAVA® IV, and watch the How RADICAVA® May Help video.
Introduction to RADICAVA® for Newly Diagnosed Patients
Sunny and RADICAVA® IV: A Personal Infusion Experience
Sunny Brous, a real-life patient with years of experience on RADICAVA®, talks about the infusion process and how she has incorporated it into her daily routine
See more about receiving a RADICAVA® IV infusion,
and watch the Administering RADICAVA® IV video.
Sunny and RADICAVA® IV: A Personal Infusion Experience
Videos are sponsored by Mitsubishi Tanabe Pharma America, Inc. (MTPA). The speakers are presenting on behalf of, and have received compensation from MTPA.
Webinars are intended for general information purposes only and are not a substitute for professional medical advice, diagnosis, or treatment. Patients are always encouraged to talk to their doctor or other qualified healthcare provider with any questions regarding a medical condition.
It is important to discuss any insurance-related questions with your healthcare provider and insurance provider.
Please see Important Safety Information below and click here for full Prescribing Information and Patient Information.
Hear from other PALS and CALS
Watch real peoples' videos and read written stories about their experiences with ALS and RADICAVA®.
Have questions about RADICAVA® and ALS?
A JourneyMate Resource Specialist is ready to help you find information and educational resources about ALS and RADICAVA® as you move forward on your journey with ALS. Call toll-free 1-855-457-6968 between 9 AM and 9 PM ET, Monday through Friday.
Learn more
